- Barajar
ActivarDesactivar
- Alphabetizar
ActivarDesactivar
- Frente Primero
ActivarDesactivar
- Ambos lados
ActivarDesactivar
- Leer
ActivarDesactivar
Leyendo...
Cómo estudiar sus tarjetas
Teclas de Derecha/Izquierda: Navegar entre tarjetas.tecla derechatecla izquierda
Teclas Arriba/Abajo: Colvea la carta entre frente y dorso.tecla abajotecla arriba
Tecla H: Muestra pista (3er lado).tecla h
Tecla N: Lea el texto en voz.tecla n
![]()
Boton play
![]()
Boton play
![]()
3 Cartas en este set
- Frente
- Atrás
- 3er lado (pista)
|
dalton's ideas about matter
|
-the elements are made up of discretes particles of matter(atoms) which are indivisible and inarterable
-all the atoms of a single element are same size and mass -compounds are formed when tge atoms of the differents elements join together -in a chemical reaction, the atoms regroup, such that they are aggared differently how they were before. however,there are not created or destroyed. |
4 points
|
|
the law of conservation of mass
|
-in a chemical reaction matter is neither created not destroyed, but simply transform
-the mass of the subtances that start the reactis the same as mass of the subtances produced |
|
|
examples of conservation of mass
|
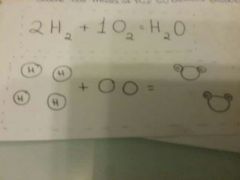
|